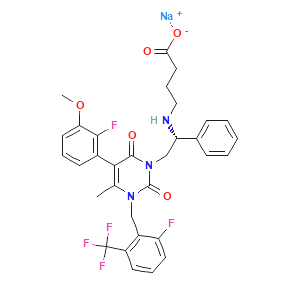
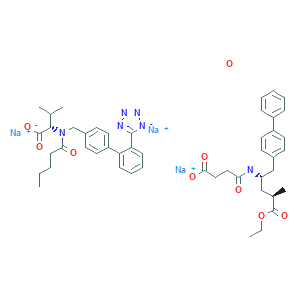
| Name: | Elagolix sodium |
|---|---|
| CAS:: | 832720-36-2 |
| Molecular Formula: | C32H29F5N3NaO5 |
| Standard: | In-house |
| Documentation: | CDMF |
| Development stage: | Commercialization |
Related Products
Elagolix sodium
Elagolix, sold under the brand name Orilissa, is a gonadotropin-releasing hormone antagonist (GnRH antagonist) medication which is used in the treatment of pain associated with endometriosis in women. It is also under development for the treatment of uterine fibroids and heavy menstrual bleeding in women.The medication was under investigation for the treatment of prostate cancer and enlarged prostate in men as well, but development for these conditions was discontinued. Elagolix is taken by mouth once or twice per day. It can be taken for up to 6 to 24 months, depending on the dosage.
Side effects of elagolix include menopausal-like symptoms such as hot flashes, night sweats, insomnia, amenorrhea, mood changes, anxiety, and decreased bone density, among others. Elagolix is a GnRH antagonist, or an antagonist of the gonadotropin-releasing hormone receptor (GnRHR), the biological target of the hypothalamic hormone gonadotropin-releasing hormone (GnRH). By blocking the GnRHR, it dose-dependently suppresses the gonadal production and hence circulating levels of sex hormones such as estradiol, progesterone, and testosterone.Elagolix is a short-acting GnRH antagonist, and can be used to achieve either partial or more substantial suppression of sex hormone levels.Reduced estrogen levels in the endometrium are responsible for the efficacy of elagolix in the treatment of endometriosis.