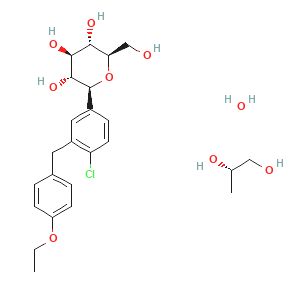
| Name: | Dapagliflozin propanediol monohydrate |
|---|---|
| CAS:: | 960404-48-2 |
| Molecular Formula: | C24H35ClO9 |
| Standard: | In-house |
| Documentation: | CDMF |
| Development stage: | Commercialization |
Related Products
Dapagliflozin propanediol monohydrate
Dapagliflozin, sold under the brand names Farxiga (US) and Forxiga (EU) among others, is a medication used to treat type 2 diabetes. It is also used to treat adults with heart failure and chronic kidney disease.
Common side effects include hypoglycaemia (low blood sugar), urinary tract infections, genital infections, and volume depletion (reduced amount of water in the body). Diabetic ketoacidosis is a common side effect in type 1 diabetic patients.Serious but rare side effects include Fournier gangrene.Dapagliflozin is a sodium-glucose co-transporter-2 (SGLT-2) inhibitor and works by removing sugar from the body with the urine.
It was developed by Bristol-Myers Squibb in partnership with AstraZeneca. It is on the World Health Organization's List of Essential Medicines. In 2020, it was the 217th-most commonly prescribed medication in the United States, with more than 2 million prescriptions.