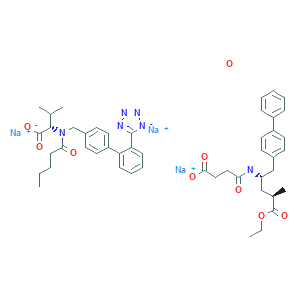
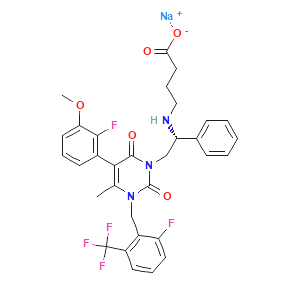
| Name: | Sacubitril/valsartan(LCZ 696) |
|---|---|
| CAS:: | 936623-90-4 |
| Molecular Formula: | C96H120N12Na6O21 |
| Standard: | In-house |
| Documentation: | CDMF |
| Development stage: | Commercialization |
Related Products
Sacubitril/valsartan
Sacubitril/valsartan, sold under the brand name Entresto, is a fixed-dose combination medication for use in heart failure. It consists of the neprilysin inhibitor sacubitril and the angiotensin receptor blocker valsartan. The combination is sometimes described as an "angiotensin receptor-neprilysin inhibitor" (ARNi).It is recommended for use as a replacement for an ACE inhibitor or an angiotensin receptor blocker in people with heart failure with reduced ejection fraction.
Potential side effects include angioedema, kidney problems, and low blood pressure.
It was approved for medical use in the United States and in the European Union in 2015, and in Australia in 2016. In 2020, it was the 219th most commonly prescribed medication in the United States, with more than 2 million prescriptions.